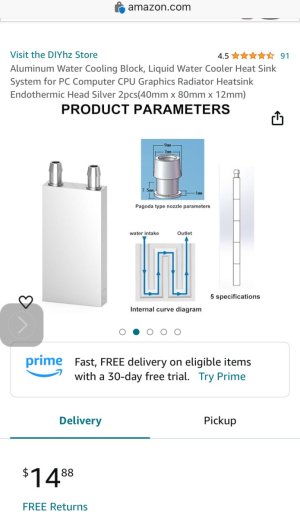
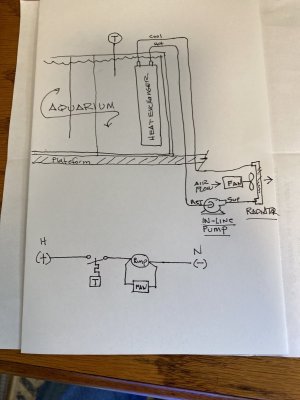
I thought there was a discussion about cooling tank water on this site, maybe not.
Now there is, eh?
I don’t have the issue of cooling my tank water, yet. I came across this equipment while down another rabbit hole looking for something else and thought I needed to throw it out there before I forget.
I have no idea if this will work. My gut says yes. I would have to do some mechanical engineering math for myself to figure it out. You can too.
https://a.co/d/exMTCoW
Follow this link on Amazon, and you will find the photo below too.
It might be worth checking out.
Now there is, eh?
I don’t have the issue of cooling my tank water, yet. I came across this equipment while down another rabbit hole looking for something else and thought I needed to throw it out there before I forget.
I have no idea if this will work. My gut says yes. I would have to do some mechanical engineering math for myself to figure it out. You can too.
https://a.co/d/exMTCoW
Follow this link on Amazon, and you will find the photo below too.
It might be worth checking out.